Which Copper-zinc Brazing Alloy Could Be Used To Join Or Repair Each Of The Following

Brazing is a metal-joining process in which two or more than metal items are joined together past melting and flowing a filler metal into the articulation, with the filler metal having a lower melting point than the adjoining metal.
Brazing differs from welding in that it does not involve melting the work pieces. Brazing differs from soldering through the use of a higher temperature and much more closely fitted parts than when soldering. During the brazing procedure, the filler metal flows into the gap between close-fitting parts past capillary action. The filler metal is brought slightly higher up its melting (liquidus) temperature while protected by a suitable atmosphere, usually a flux. It then flows over the base metallic (in a process known equally wetting) and is then cooled to join the piece of work pieces together.[1] A major reward of brazing is the ability to join the same or different metals with considerable strength.
Nuts [edit]
High-quality brazed joints require that parts be closely fitted with base metal surfaces exceptionally clean and costless of oxides. In near cases, joint clearances of 0.03 to 0.08 mm (0.0012 to 0.0031 in) are recommended for the best capillary activeness and joint force;[2] in some brazing operations, even so, it is not uncommon to have articulation clearances around 0.vi mm (0.024 in). Cleanliness of the brazing surfaces is also important, as whatever contamination can cause poor wetting (flow). The 2 main methods for cleaning parts, prior to brazing, are chemical cleaning and annoying or mechanical cleaning. In the instance of mechanical cleaning information technology is of import to maintain the proper surface roughness, as wetting on a rough surface occurs much more readily than on a smooth surface of the same geometry.[2]
Some other consideration is the event of temperature and time on the quality of brazed joints. Every bit the temperature of the braze alloy is increased, the alloying and wetting action of the filler metallic increases too. In general, the brazing temperature selected must be above the melting point of the filler metal. Still, several factors influence the articulation designer's temperature selection. The best temperature is normally selected to:
- Minimize braze temperature
- Minimize any estrus effects on the assembly
- Minimize filler metallic/base metal interaction
- Maximize the life of whatever fixtures or jigs used[2]
In some cases, a worker may select a higher temperature to accommodate other factors in the blueprint (east.thou., to let utilize of a different filler metallic, or to command metallurgical effects, or to sufficiently remove surface contamination). The effect of time on the brazed joint primarily affects the extent to which these effects are present. In general, notwithstanding, most product processes are selected to minimize brazing fourth dimension and associated costs. This is not always the instance, all the same, since in some not-production settings, time and cost are secondary to other joint attributes (due east.one thousand., strength, appearance).
Flux [edit]
Unless brazing operations are contained inside an inert or reducing atmosphere environment (i.e. Nitrogen), a flux such every bit borax is required to prevent oxides from forming while the metallic is heated. The flux as well serves the purpose of cleaning any contagion left on the brazing surfaces. Flux tin can be applied in any number of forms including flux paste, liquid, pulverisation or pre-made brazing pastes that combine flux with filler metal pulverisation. Flux can also be applied using brazing rods with a blanket of flux, or a flux cadre. In either case, the flux flows into the articulation when applied to the heated joint and is displaced by the molten filler metal entering the joint. Backlog flux should be removed when the bicycle is completed because flux left in the articulation tin lead to corrosion, impede joint inspection, and prevent farther surface finishing operations. Phosphorus-containing brazing alloys can be self-fluxing when joining copper to copper.[iii] Fluxes are generally selected based on their performance on particular base metals. To be effective, the flux must be chemically compatible with both the base of operations metal and the filler metal existence used. Cocky-fluxing phosphorus filler alloys produce brittle phosphides if used on fe or nickel.[three] As a general rule, longer brazing cycles should use less active fluxes than short brazing operations.[4]
Filler materials [edit]
A variety of alloys are used as filler metals for brazing depending on the intended use or awarding method. In full general, braze alloys are made up of 3 or more metals to class an alloy with the desired properties. The filler metallic for a item application is chosen based on its ability to: moisture the base metals, withstand the service weather condition required, and melt at a lower temperature than the base metals or at a very specific temperature.
Braze alloy is generally bachelor as rod, ribbon, powder, paste, cream, wire and preforms (such as stamped washers).[v] Depending on the application, the filler material can be pre-placed at the desired location or applied during the heating cycle. For manual brazing, wire and rod forms are by and large used equally they are the easiest to apply while heating. In the example of furnace brazing, alloy is usually placed beforehand since the process is usually highly automated.[v] Some of the more mutual types of filler metals used are
- Aluminum-silicon
- Copper
- Copper-silver
- Copper-zinc (brass)
- Copper-tin (bronze)
- Gold-silvery
- Nickel blend
- Silver[1] [6]
- Baggy brazing foil using nickel, iron, copper, silicon, boron, phosphorus, etc.
Temper [edit]
As brazing work requires high temperatures, oxidation of the metal surface occurs in an oxygen-containing atmosphere. This may necessitate the use of an atmospheric environment other than air. The commonly used atmospheres are[7] [eight]
- Air: Simple and economical. Many materials susceptible to oxidation and buildup of calibration. Acrid cleaning bath or mechanical cleaning can exist used to remove the oxidation afterward work. Flux counteracts the oxidation, simply may weaken the articulation.
- Combusted fuel gas (low hydrogen, AWS type ane, "exothermic generated atmospheres"): 87% Northtwo, 11–12% CO2, 5-ane% CO, five-1% H2. For silver, copper-phosphorus and copper-zinc filler metals. For brazing copper and brass.
- Combusted fuel gas (decarburizing, AWS type ii, "endothermic generated atmospheres"): seventy–71% N2, 5–vi% COtwo, nine–10% CO, xiv–fifteen% H2. For copper, silver, copper-phosphorus and copper-zinc filler metals. For brazing copper, brass, nickel alloys, Monel, medium carbon steels.
- Combusted fuel gas (dried, AWS type 3, "endothermic generated atmospheres"): 73–75% Ntwo, 10–eleven% CO, fifteen–16% H2. For copper, silver, copper-phosphorus and copper-zinc filler metals. For brazing copper, brass, low-nickel alloys, Monel, medium and high carbon steels.
- Combusted fuel gas (dried, decarburizing, AWS type four): 41–45% Ntwo, 17–19% CO, 38–forty% H2. For copper, silver, copper-phosphorus and copper-zinc filler metals. For brazing copper, brass, low-nickel alloys, medium and high carbon steels.
- Ammonia (AWS type v, also chosen forming gas): Dissociated ammonia (75% hydrogen, 25% nitrogen) can be used for many types of brazing and annealing. Inexpensive. For copper, silvery, nickel, copper-phosphorus and copper-zinc filler metals. For brazing copper, brass, nickel alloys, Monel, medium and loftier carbon steels and chromium alloys.
- Nitrogen+hydrogen, cryogenic or purified (AWS blazon 6A): seventy–99% Ntwo, i–30% H2. For copper, silver, nickel, copper-phosphorus and copper-zinc filler metals.
- Nitrogen+hydrogen+carbon monoxide, cryogenic or purified (AWS type 6B): 70–99% Nii, ii–twenty% Hii, 1–10% CO. For copper, silverish, nickel, copper-phosphorus and copper-zinc filler metals. For brazing copper, brass, low-nickel alloys, medium and high carbon steels.
- Nitrogen, cryogenic or purified (AWS type 6C): Non-oxidizing, economical. At high temperatures can react with some metals, eastward.m. certain steels, forming nitrides. For copper, silver, nickel, copper-phosphorus and copper-zinc filler metals. For brazing copper, brass, low-nickel alloys, Monel, medium and high carbon steels.
- Hydrogen (AWS type seven): Potent deoxidizer, highly thermally conductive. Tin exist used for copper brazing and annealing steel. May cause hydrogen embrittlement to some alloys. For copper, silver, nickel, copper-phosphorus and copper-zinc filler metals. For brazing copper, brass, nickel alloys, Monel, medium and high carbon steels and chromium alloys, cobalt alloys, tungsten alloys, and carbides.
- Inorganic vapors (various volatile fluorides, AWS type 8): Special purpose. Tin be mixed with atmospheres AWS one–5 to supersede flux. Used for silver-brazing of brasses.
- Noble gas (usually argon, AWS type 9): Not-oxidizing, more expensive than nitrogen. Inert. Parts must be very clean, gas must exist pure. For copper, silver, nickel, copper-phosphorus and copper-zinc filler metals. For brazing copper, brass, nickel alloys, Monel, medium and high carbon steels chromium alloys, titanium, zirconium, hafnium.
- Element of group 0+hydrogen (AWS type 9A)
- Vacuum: Requires evacuating the work sleeping room. Expensive. Unsuitable (or requires special intendance) for metals with high vapor force per unit area, east.g. silver, zinc, phosphorus, cadmium, and manganese. Used for highest-quality joints, for due east.g. aerospace applications.
Common techniques [edit]
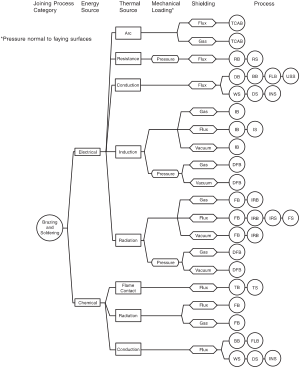
Torch brazing [edit]
Torch brazing is by far the most common method of mechanized brazing in use. It is best used in small production volumes or in specialized operations, and in some countries, information technology accounts for a majority of the brazing taking place. There are three chief categories of torch brazing in use:[ten] transmission, machine, and automatic torch brazing.
Manual torch brazing is a procedure where the rut is applied using a gas flame placed on or near the joint being brazed. The torch tin either be hand held or held in a fixed position depending on whether the operation is completely transmission or has some level of automation. Manual brazing is about commonly used on small production volumes or in applications where the part size or configuration makes other brazing methods impossible.[10] The main drawback is the high labor cost associated with the method as well every bit the operator skill required to obtain quality brazed joints. The apply of flux or self-fluxing material is required to prevent oxidation. Torch brazing of copper can be done without the utilize of flux if it is brazed with a torch using oxygen and hydrogen gas, rather than oxygen and other flammable gases.
Machine torch brazing is ordinarily used where a repetitive braze performance is being carried out. This method is a mix of both automated and manual operations with an operator often placing brazes fabric, flux and jigging parts while the machine mechanism carries out the actual braze.[10] The advantage of this method is that it reduces the high labor and skill requirement of manual brazing. The use of flux is also required for this method as at that place is no protective temper, and information technology is best suited to small to medium product volumes.
Automated torch brazing is a method that almost eliminates the need for transmission labor in the brazing operation, except for loading and unloading of the machine. The main advantages of this method are: a high production rate, uniform braze quality, and reduced operating toll. The equipment used is substantially the same as that used for Auto torch brazing, with the main difference beingness that the machinery replaces the operator in the role preparation.[ten]
Furnace brazing [edit]
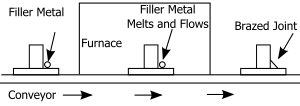
Furnace brazing schematic
Furnace brazing is a semi-automatic process used widely in industrial brazing operations due to its adaptability to mass production and apply of unskilled labor. In that location are many advantages of furnace brazing over other heating methods that make it ideal for mass production. One principal advantage is the ease with which information technology tin can produce large numbers of pocket-sized parts that are easily jigged or cocky-locating.[11] The procedure also offers the benefits of a controlled heat cycle (assuasive use of parts that might distort under localized heating) and no need for post braze cleaning. Mutual atmospheres used include: inert, reducing or vacuum atmospheres all of which protect the part from oxidation. Some other advantages include: low unit price when used in mass product, close temperature control, and the ability to braze multiple joints at once. Furnaces are typically heated using either electric, gas or oil depending on the type of furnace and application. However, some of the disadvantages of this method include: high capital equipment toll, more difficult blueprint considerations and high power consumption.[11]
At that place are four principal types of furnaces used in brazing operations: batch type; continuous; retort with controlled atmosphere; and vacuum.
A batch type furnace has relatively low initial equipment costs, and can heat each office load separately. It can turned on and off at will, which reduces operating expenses when information technology's not in use. These furnaces are suited to medium to large book product, and offer a large degree of flexibility in type of parts that tin can exist brazed.[11] Either controlled atmospheres or flux can be used to control oxidation and cleanliness of parts.
Continuous type furnaces are best suited to a steady flow of similar-sized parts through the furnace.[11] These furnaces are oft conveyor fed, moving parts through the hot zone at a controlled speed. It is common to utilize either controlled temper or pre-applied flux in continuous furnaces. In item, these furnaces offer the benefit of very depression manual labor requirements and so are all-time suited to large scale product operations.
Retort-blazon furnaces differ from other batch-type furnaces in that they brand apply of a sealed lining called a "retort". The retort is mostly sealed with either a gasket or is welded shut and filled completely with the desired atmosphere and then heated externally by conventional heating elements.[xi] Due to the high temperatures involved, the retort is usually made of oestrus resistant alloys that resist oxidation. Retort furnaces are often either used in a batch or semi-continuous versions[ dubious ].
Vacuum furnaces is a relatively economical method of oxide prevention and is most often used to braze materials with very stable oxides (aluminum, titanium and zirconium) that cannot be brazed in atmosphere furnaces. Vacuum brazing is also used heavily with refractory materials and other exotic blend combinations unsuited to atmosphere furnaces. Due to the absence of flux or a reducing atmosphere, the part cleanliness is disquisitional when brazing in a vacuum. The iii principal types of vacuum furnace are: single-wall hot retort, double-walled hot retort, and common cold-wall antiphon. Typical vacuum levels for brazing range from pressures of 1.iii to 0.thirteen pascals (ten−ii to 10−three Torr) to 0.00013 Pa (10−6 Torr) or lower.[eleven] Vacuum furnaces are almost commonly batch-type, and they are suited to medium and high production volumes.
Silver brazing [edit]
Silver brazing , sometimes known every bit a hard soldering , is brazing using a silver alloy based filler. These silver alloys consist of many different percentages of silverish and other metals, such as copper, zinc and cadmium.

Crack in ninety–x Cu–Ni metal plate due to stresses during silverish brazing
Brazing is widely used in the tool industry to fasten "hard metal" (carbide, ceramics, cermet, and similar) tips to tools such as saw blades. "Pretinning" is oft done: the affix alloy is melted onto the hard metal tip, which is placed next to the steel and remelted. Pretinning gets effectually the trouble that hard metals are difficult to wet.
Brazed difficult metal joints are typically two to vii mils thick. The braze alloy joins the materials and compensates for the difference in their expansion rates. It besides provides a cushion between the hard carbide tip and the difficult steel, which softens touch and prevents tip loss and damage—much equally a vehicle'southward suspension helps foreclose damage to the tires and the vehicle. Finally, the braze alloy joins the other two materials to create a composite structure, much as layers of wood and gum create plywood. The standard for braze joint strength in many industries is a joint that is stronger than either base material, then that when under stress, i or other of the base materials fails earlier the articulation. Silver brazing may crusade defects in certain alloys, eastward.g. stress-induced inter-granular smashing in copper-nickel.
One special silver brazing method is called pinbrazing or pin brazing . It has been developed specially for connecting cables to railway track or for cathodic protection installations. The method uses a silver- and flux-containing brazing pin, which is melted in the heart of a cable lug. The equipment is unremarkably powered from batteries.
Braze welding [edit]
Affix welding is the use of a bronze or brass filler rod coated with flux to join steel workpieces. The equipment needed for braze welding is basically identical to the equipment used in brazing. Since braze welding commonly requires more heat than brazing, acetylene or methylacetylene-propadiene (MAP) gas fuel is commonly used. The proper name comes from the fact that no capillary action is used.
Affix welding has many advantages over fusion welding. It allows the joining of dissimilar metals, minimization of estrus distortion, and can reduce the need for all-encompassing pre-heating. Additionally, since the metals joined are not melted in the process, the components retain their original shape; edges and contours are not eroded or changed by the formation of a fillet. Some other effect of affix welding is the elimination of stored-up stresses that are oft nowadays in fusion welding. This is extremely of import in the repair of large castings. The disadvantages are the loss of strength when subjected to high temperatures and the disability to withstand high stresses.
Carbide, cermet and ceramic tips are plated and then joined to steel to brand tipped band saws. The plating acts as a braze alloy.
Cast atomic number 26 "welding" [edit]
The "welding" of bandage iron is usually a brazing operation, with a filler rod made importantly of nickel existence used although true welding with bandage iron rods is also available. Ductile cast iron pipe may be also "cadwelded," a process that connects joints by means of a small copper wire fused into the iron when previously ground down to the bare metallic, parallel to the atomic number 26 joints being formed as per hub pipe with neoprene gasket seals. The purpose behind this performance is to utilise electricity along the copper for keeping underground pipes warm in cold climates.
Vacuum brazing [edit]
Vacuum brazing is a material joining technique that offers pregnant advantages: extremely clean, superior, flux-gratis affix joints of high integrity and strength. The process can be expensive because it must be performed within a vacuum chamber vessel. Temperature uniformity is maintained on the work slice when heating in a vacuum, greatly reducing residual stresses due to dull heating and cooling cycles. This, in turn, tin can significantly improve the thermal and mechanical properties of the cloth, thus providing unique heat treatment capabilities. Ane such capability is rut-treating or age-hardening the workpiece while performing a metallic-joining process, all in a unmarried furnace thermal cycle.
Products that are well-nigh commonly vacuum-brazed include aluminum cold plates, plate-fin estrus exchangers, and flat tube estrus exchangers.[12]
Vacuum brazing is often conducted in a furnace; this ways that several joints tin can be made at once because the whole workpiece reaches the brazing temperature. The heat is transferred using radiation, every bit many other methods cannot be used in a vacuum.
Dip brazing [edit]
Dip brazing is specially suited for brazing aluminium because air is excluded, thus preventing the germination of oxides. The parts to be joined are fixtured and the brazing compound applied to the mating surfaces, typically in slurry form. Then the assemblies are dipped into a bathroom of molten common salt (typically NaCl, KCl and other compounds), which functions equally both heat transfer medium and flux. Many dip brazed parts are used in rut transfer applications for the aerospace industry.[13]
Heating methods [edit]
| | This section needs expansion. You can aid past adding to it. (September 2009) |

A Us Navy maintenance technician torch brazes a steel piping
There are many heating methods available to accomplish brazing operations. The most important factor in choosing a heating method is achieving efficient transfer of rut throughout the articulation and doing so inside the oestrus capacity of the private base of operations metals used. The geometry of the braze joint is also a crucial factor to consider, as is the rate and volume of production required. The easiest way to categorize brazing methods is to grouping them by heating method. Here are some of the virtually common:[ane] [fourteen]
- Torch brazing
- Furnace brazing
- Induction brazing
- Dip brazing
- Resistance brazing
- Infrared brazing
- Coating brazing
- Electron axle and laser brazing
- Affix welding
These heating methods are classified through localised and diffuse heating techniques and offering advantages based on their unlike applications.[15]
Condom [edit]
Brazing may entail exposure to hazardous chemical fumes. The National Institute for Occupational Rubber and Health in the United states recommends that exposure to these fumes is controlled to levels below the immune exposure limit.[16]
Advantages and disadvantages [edit]
Brazing has many advantages over other metallic-joining techniques, such as welding. Since brazing does not cook the base metal of the joint, it allows much tighter control over tolerances and produces a clean joint without the need for secondary finishing. Additionally, dissimilar metals and non-metals (i.due east. metalized ceramics) can exist brazed.[17] In full general, brazing also produces less thermal baloney than welding due to the uniform heating of a brazed piece. Complex and multi-function assemblies can be brazed cost-finer. Welded joints must sometimes exist ground flush, a plush secondary operation that brazing does not require because it produces a clean joint. Some other reward is that the brazing can be coated or clad for protective purposes. Finally, brazing is easily adapted to mass production and it is easy to automate considering the private process parameters are less sensitive to variation.[18] [19]
I of the master disadvantages is the lack of joint force every bit compared to a welded joint due to the softer filler metals used.[1] The strength of the brazed articulation is probable to be less than that of the base metal(s) but greater than the filler metal.[twenty] Another disadvantage is that brazed joints can exist damaged under high service temperatures.[i] Brazed joints require a high degree of base-metallic cleanliness when done in an industrial setting. Some brazing applications crave the use of acceptable fluxing agents to control cleanliness. The articulation colour is often different from that of the base metal, creating an aesthetic disadvantage.
Filler metals [edit]
Some brazes come in the form of trifoils, laminated foils of a carrier metal clad with a layer of braze at each side. The center metal is oftentimes copper; its role is to human action every bit a carrier for the alloy, to absorb mechanical stresses due to east.yard. differential thermal expansion of dissimilar materials (eastward.grand. a carbide tip and a steel holder), and to act as a diffusion barrier (e.yard. to finish diffusion of aluminium from aluminium statuary to steel when brazing these ii).
Braze families [edit]
Brazing alloys grade several distinct groups; the alloys in the same group have similar backdrop and uses.[21]
- Pure metals
- Unalloyed. Often noble metals – silver, gold, palladium.
- Ag-Cu
- Silvery-copper. Good melting properties. Silvery enhances menstruum. Eutectic alloy used for furnace brazing. Copper-rich alloys decumbent to stress bully past ammonia.
- Ag-Zn
- Silver-zinc. Similar to Cu-Zn, used in jewelry due to its high silverish content so that the production is compliant with hallmarking. The colour matches argent, and it is resistant to ammonia-containing silver-cleaning fluids.
- Cu-Zn (brass)
- Copper-zinc. General purpose, used for joining steel and bandage iron. Corrosion resistance usually inadequate for copper, silicon bronze, copper-nickel, and stainless steel. Reasonably ductile. High vapor pressure due to volatile zinc, unsuitable for furnace brazing. Copper-rich alloys prone to stress cracking by ammonia.
- Ag-Cu-Zn
- Silver-copper-zinc. Lower melting point than Ag-Cu for same Ag content. Combines advantages of Ag-Cu and Cu-Zn. At above twoscore% Zn the ductility and strength drop, so just lower-zinc alloys of this blazon are used. At above 25% zinc less ductile copper-zinc and silverish-zinc phases appear. Copper content higher up 60% yields reduced strength and liquidus above 900 °C. Silver content above 85% yields reduced strength, high liquidus and loftier price. Copper-rich alloys prone to stress groovy past ammonia. Silvery-rich brazes (to a higher place 67.5% Ag) are hallmarkable and used in jewellery; alloys with lower silver content are used for technology purposes. Alloys with copper-zinc ratio of about 60:40 incorporate the aforementioned phases as brass and match its color; they are used for joining brass. Small amount of nickel improves strength and corrosion resistance and promotes wetting of carbides. Improver of manganese together with nickel increases fracture toughness. Add-on of cadmium yields Ag-Cu-Zn-Cd alloys with improved fluidity and wetting and lower melting point; however cadmium is toxic. Improver of tin can play mostly the aforementioned role.
- Cu-P
- Copper-phosphorus. Widely used for copper and copper alloys. Does not require flux for copper. Can be besides used with silver, tungsten, and molybdenum. Copper-rich alloys decumbent to stress bully by ammonia.
- Ag-Cu-P
- Like Cu-P, with improved catamenia. Better for larger gaps. More than ductile, improve electrical electrical conductivity. Copper-rich alloys prone to stress cracking by ammonia.
- Au-Ag
- Golden-silverish. Noble metals. Used in jewelry.
- Au-Cu
- Golden-copper. Continuous serial of solid solutions. Readily wet many metals, including refractory ones. Narrow melting ranges, skillful fluidity.[22] Frequently used in jewellery. Alloys with forty–90% of gold harden on cooling but stay ductile. Nickel improves ductility. Silver lowers melting indicate but worsens corrosion resistance. To maintain corrosion resistance, gold must exist kept above lx%. High-temperature strength and corrosion resistance can exist improved past further alloying, e.one thousand., with chromium, palladium, manganese, and molybdenum. Added vanadium allows wetting ceramics. Gilt-copper has low vapor pressure.
- Au-Ni
- Gold-Nickel. Continuous series of solid solutions. Wider melting range than Au-Cu alloys but meliorate corrosion resistance and improved wetting. Ofttimes alloyed with other metals to reduce proportion of gold while maintaining properties. Copper may be added to lower golden proportion, chromium to compensate for loss of corrosion resistance, and boron for improving wetting impaired by the chromium. More often than not no more than 35% Ni is used, as higher Ni/Au ratios have likewise wide melting range. Depression vapor pressure.
- Au-Pd
- Gold-Palladium. Improved corrosion resistance over Au-Cu and Au-Ni alloys. Used for joining superalloys and refractory metals for loftier-temperature applications, due east.g. jet engines. Expensive. May be substituted for past cobalt-based brazes. Low vapor pressure.
- Pd
- Palladium. Good high-temperature performance, high corrosion resistance (less than gold), high strength (more than gold). usually alloyed with nickel, copper, or silver. Forms solid solutions with virtually metals, does non grade brittle intermetallics. Low vapor pressure.
- Ni
- Nickel alloys, even more than numerous than argent alloys. High force. Lower price than silver alloys. Proficient high-temperature performance, good corrosion resistance in moderately aggressive environments. Ofttimes used for stainless steels and estrus-resistant alloys. Embrittled with sulfur and some lower-melting bespeak metals, e.thou. zinc. Boron, phosphorus, silicon and carbon lower melting point and quickly diffuse to base metals. This allows diffusion brazing, and lets the articulation be used above the brazing temperature. Borides and phosphides form brittle phases. Amorphous preforms can be fabricated by rapid solidification.
- Co
- Cobalt alloys. Practiced high-temperature corrosion resistance, possible alternative to Au-Pd brazes. Low workability at low temperatures, preforms prepared past rapid solidification.
- Al-Si
- Aluminium-silicon. For brazing aluminium.
- Active alloys
- Containing active metals, east.1000., titanium or vanadium. Used for brazing non-metallic materials, due east.grand. graphite or ceramics.
Role of elements [edit]
| element | role | volatility | corrosion resistance | cost | incompatibility | description |
|---|---|---|---|---|---|---|
| Silvery | structural, wetting | volatile | expensive | Enhances capillary flow, improves corrosion resistance of less-noble alloys, worsens corrosion resistance of gold and palladium. Relatively expensive. High vapor pressure, problematic in vacuum brazing. Wets copper. Does not wet nickel and iron. Reduces melting betoken of many alloys, including gold-copper. | ||
| Copper | structural | ammonia | Good mechanical properties. Often used with silver. Dissolves and wets nickel. Somewhat dissolves and wets atomic number 26. Copper-rich alloys sensitive to stress slap-up in presence of ammonia. | |||
| Zinc | structural, melting, wetting | volatile | low | cheap | Ni | Lowers melting point. Ofttimes used with copper. Susceptible to corrosion. Improves wetting on ferrous metals and on nickel alloys. Compatible with aluminium. Loftier vapor tension, produces somewhat toxic fumes, requires ventilation; highly volatile higher up 500 °C. At high temperatures may boil and create voids. Prone to selective leaching in some environments, which may cause joint failure. Traces of bismuth and beryllium together with tin or zinc in aluminium-based braze destabilize oxide film on aluminium, facilitating its wetting. High affinity to oxygen, promotes wetting of copper in air past reduction of the cuprous oxide surface film. Less such benefit in furnace brazing with controlled atmosphere. Embrittles nickel. High levels of zinc may outcome in a breakable blend.[23] Prone to interfacial corrosion in contact with stainless steel in wet and humid environments. Unsuitable for furnace brazing due to volatility. |
| Aluminium | structural, agile | Iron | Usual base for brazing aluminium and its alloys. Embrittles ferrous alloys. | |||
| Golden | structural, wetting | fantabulous | very expensive | Excellent corrosion resistance. Very expensive. Wets most metals. | ||
| Palladium | structural | splendid | very expensive | First-class corrosion resistance, though less than aureate. Higher mechanical strength than golden. Good loftier-temperature strength. Very expensive, though less than aureate. Makes the articulation less prone to fail due to intergranular penetration when brazing alloys of nickel, molybdenum, or tungsten.[24] Increases high-temperature strength of gilded-based alloys.[22] Improves loftier-temperature strength and corrosion resistance of gold-copper alloys. Forms solid solutions with most engineering metals, does not form brittle intermetallics. Loftier oxidation resistance at high temperatures, peculiarly Pd-Ni alloys. | ||
| Cadmium | structural, wetting, melting | volatile | toxic | Lowers melting point, improves fluidity. Toxic. Produces toxic fumes, requires ventilation. Loftier affinity to oxygen, promotes wetting of copper in air by reduction of the cuprous oxide surface moving picture. Less such do good in furnace brazing with controlled atmosphere. Allows reducing silver content of Ag-Cu-Zn alloys. Replaced past can in more modern alloys. In EU since December 2011 allowed only for aerospace and military use.[25] | ||
| Lead | structural, melting | Lowers melting point. Toxic. Produces toxic fumes, requires ventilation. | ||||
| Tin | structural, melting, wetting | Lowers melting point, improves fluidity. Broadens melting range. Tin can be used with copper, with which information technology forms bronze. Improves wetting of many difficult-to-wet metals, eastward.grand. stainless steels and tungsten carbide. Traces of bismuth and beryllium together with tin or zinc in aluminium-based braze destabilize oxide motion-picture show on aluminium, facilitating its wetting. Low solubility in zinc, which limits its content in zinc-bearing alloys.[23] | ||||
| Bismuth | trace condiment | Lowers melting point. May disrupt surface oxides. Traces of bismuth and beryllium together with tin or zinc in aluminium-based affix destabilize oxide film on aluminium, facilitating its wetting.[23] | ||||
| Beryllium | trace additive | toxic | Traces of bismuth and beryllium together with tin or zinc in aluminium-based braze destabilize oxide film on aluminium, facilitating its wetting.[23] | |||
| Nickel | structural, wetting | loftier | Zn, Southward | Strong, corrosion-resistant. Impedes flow of the melt. Add-on to gilded-copper alloys improves ductility and resistance to pitter-patter at high temperatures.[22] Addition to silver allows wetting of silverish-tungsten alloys and improves bond strength. Improves wetting of copper-based brazes. Improves ductility of gold-copper brazes. Improves mechanical properties and corrosion resistance of silvery-copper-zinc brazes. Nickel content offsets brittleness induced past diffusion of aluminium when brazing aluminium-containing alloys, e.g. aluminium bronzes. In some alloys increases mechanical properties and corrosion resistance, by a combination of solid solution strengthening, grain refinement, and segregation on fillet surface and in grain boundaries, where it forms a corrosion-resistant layer. Extensive intersolubility with iron, chromium, manganese, and others; tin can severely erode such alloys. Embrittled by zinc, many other low melting bespeak metals, and sulfur.[23] | ||
| Chromium | structural | high | Corrosion-resistant. Increases loftier-temperature corrosion resistance and strength of gold-based alloys. Added to copper and nickel to increase corrosion resistance of them and their alloys.[22] Wets oxides, carbides, and graphite; frequently a major alloy component for high-temperature brazing of such materials. Impairs wetting by gold-nickel alloys, which can be compensated for by addition of boron.[23] | |||
| Manganese | structural | volatile | good | cheap | Loftier vapor pressure, unsuitable for vacuum brazing. In gold-based alloys increases ductility. Increases corrosion resistance of copper and nickel alloys.[22] Improves loftier-temperature strength and corrosion resistance of gold-copper alloys. Higher manganese content may aggravate tendency to liquation. Manganese in some alloys may tend to crusade porosity in fillets. Tends to react with graphite molds and jigs. Oxidizes hands, requires flux. Lowers melting point of loftier-copper brazes. Improves mechanical properties and corrosion resistance of silverish-copper-zinc brazes. Cheap, even less expensive than zinc. Part of the Cu-Zn-Mn system is brittle, some ratios tin not be used.[23] In some alloys increases mechanical backdrop and corrosion resistance, past a combination of solid solution strengthening, grain refinement, and segregation on fillet surface and in grain boundaries, where information technology forms a corrosion-resistant layer. Facilitates wetting of cast iron due to its power to dissolve carbon. Improves weather condition for brazing of carbides. | |
| Molybdenum | structural | good | Increases high-temperature corrosion and strength of gold-based alloys.[22] Increases ductility of gold-based alloys, promotes their wetting of refractory materials, namely carbides and graphite. When nowadays in alloys being joined, may destabilize the surface oxide layer (by oxidizing and then volatilizing) and facilitate wetting. | |||
| Cobalt | structural | good | Skillful high-temperature properties and corrosion resistance. In nuclear applications can absorb neutrons and build up cobalt-threescore, a potent gamma radiations emitter. | |||
| Magnesium | volatile Otwo getter | volatile | Addition to aluminium makes the alloy suitable for vacuum brazing. Volatile, though less than zinc. Vaporization promotes wetting past removing oxides from the surface, vapors human activity as getter for oxygen in the furnace temper. | |||
| Indium | melting, wetting | expensive | Lowers melting point. Improves wetting of ferrous alloys by copper-silver alloys. Suitable for joining parts that volition be afterwards coated past titanium nitride.[25] | |||
| Carbon | melting | Lowers melting indicate. Can grade carbides. Can lengthened to the base metallic, resulting in higher remelt temperature, potentially assuasive pace-brazing with the aforementioned blend. At above 0.1% worsens corrosion resistance of nickel alloys. Trace amounts present in stainless steel may facilitate reduction of surface chromium(Iii) oxide in vacuum and permit fluxless brazing. Diffusion abroad from the braze increases its remelt temperature; exploited in improvidence brazing.[23] | ||||
| Silicon | melting, wetting | Ni | Lowers melting point. Tin can grade silicides. Improves wetting of copper-based brazes. Promotes flow. Causes intergranular embrittlement of nickel alloys. Rapidly diffuses into the base metals. Diffusion away from the braze increases its remelt temperature; exploited in diffusion brazing. | |||
| Germanium | structural, melting | expensive | Lowers melting point. Expensive. For special applications. May create brittle phases. | |||
| Boron | melting, wetting | Ni | Lowers melting point. Tin can course hard and brittle borides. Unsuitable for nuclear reactors, as boron is a potent neutron absorber and therefore acts equally a neutron poison. Fast diffusion to the base metals. Can diffuse to the base metal, resulting in higher remelt temperature, potentially allowing step-brazing with the same alloy. Tin erode some base materials or penetrate between grain boundaries of many oestrus-resistant structural alloys, degrading their mechanical properties. Causes intergranular embrittlement of nickel alloys. Improves wetting of/by some alloys, can be added to Au-Ni-Cr alloy to compensate for wetting loss by chromium addition. In low concentrations improves wetting and lowers melting signal of nickel brazes. Quickly diffuses to base materials, may lower their melting betoken; peculiarly a business concern when brazing thin materials. Diffusion away from the braze increases its remelt temperature; exploited in diffusion brazing. | |||
| Mischmetal | trace condiment | in amount of about 0.08%, tin exist used to substitute boron where boron would have detrimental effects.[23] | ||||
| Cerium | trace additive | in trace quantities, improves fluidity of brazes. Particularly useful for alloys of four or more components, where the other additives compromise period and spreading. | ||||
| Strontium | trace additive | in trace quantities, refines the grain structure of aluminium-based alloys. | ||||
| Phosphorus | deoxidizer | H2Southward, So2, Ni, Fe, Co | Lowers melting point. Deoxidizer, decomposes copper oxide; phosphorus-bearing alloys tin be used on copper without flux. Does not decompose zinc oxide, so flux is needed for brass. Forms brittle phosphides with some metals, e.thou. nickel (Ni3P) and fe, phosphorus alloys unsuitable for brazing alloys bearing iron, nickel or cobalt in corporeality above iii%. The phosphides segregate at grain boundaries and cause intergranular embrittlement. (Sometimes the brittle joint is actually desired, though. Fragmentation grenades tin can be brazed with phosphorus bearing alloy to produce joints that shatter easily at detonation.) Avoid in environments with presence of sulfur dioxide (e.g. paper mills) and hydrogen sulfide (e.grand. sewers, or shut to volcanoes); the phosphorus-rich phase apace corrodes in presence of sulfur and the joint fails. Phosphorus can be as well present equally an impurity introduced from e.g. electroplating baths.[24] In low concentrations improves wetting and lowers melting point of nickel brazes. Diffusion abroad from the braze increases its remelt temperature; exploited in diffusion brazing. | |||
| Lithium | deoxidizer | Deoxidizer. Eliminates the need for flux with some materials. Lithium oxide formed by reaction with the surface oxides is hands displaced by molten braze alloy.[23] | ||||
| Titanium | structural, active | Most unremarkably used active metal. Few percents added to Ag-Cu alloys facilitate wetting of ceramics, eastward.grand. silicon nitride.[26] Most metals, except few (namely silver, copper and aureate), form breakable phases with titanium. When brazing ceramics, like other active metals, titanium reacts with them and forms a complex layer on their surface, which in plough is wettable by the silverish-copper braze. Wets oxides, carbides, and graphite; oft a major blend component for loftier-temperature brazing of such materials.[23] | ||||
| Zirconium | structural, active | Wets oxides, carbides, and graphite; frequently a major alloy component for high-temperature brazing of such materials.[23] | ||||
| Hafnium | active | |||||
| Vanadium | structural, active | Promotes wetting of alumina ceramics by golden-based alloys.[22] | ||||
| Sulfur | impurity | Compromises integrity of nickel alloys. Can enter the joints from residues of lubricants, grease or paint. Forms brittle nickel sulfide (Ni3S2) that segregates at grain boundaries and cause intergranular failure. |
Some additives and impurities act at very low levels. Both positive and negative effects can exist observed. Strontium at levels of 0.01% refines grain structure of aluminium. Beryllium and bismuth at like levels help disrupt the passivation layer of aluminium oxide and promote wetting. Carbon at 0.1% impairs corrosion resistance of nickel alloys. Aluminium can embrittle mild steel at 0.001%, phosphorus at 0.01%.[23]
In some cases, especially for vacuum brazing, loftier-purity metals and alloys are used. 99.99% and 99.999% purity levels are available commercially.
Care must be taken to not introduce deleterious impurities from articulation contagion or by dissolution of the base of operations metals during brazing.
Melting beliefs [edit]
Alloys with larger span of solidus/liquidus temperatures tend to melt through a "mushy" state, during which the alloy is a mixture of solid and liquid textile. Some alloys show tendency to liquation, separation of the liquid from the solid portion; for these the heating through the melting range must be sufficiently fast to avoid this event. Some alloys show extended plastic range, when only a modest portion of the alloy is liquid and nigh of the material melts at the upper temperature range; these are suitable for bridging big gaps and for forming fillets. Highly fluid alloys are suitable for penetrating deep into narrow gaps and for brazing tight joints with narrow tolerances but are not suitable for filling larger gaps. Alloys with wider melting range are less sensitive to not-uniform clearances.
When the brazing temperature is suitably loftier, brazing and heat handling can be done in a unmarried functioning simultaneously.
Eutectic alloys cook at unmarried temperature, without mushy region. Eutectic alloys have superior spreading; non-eutectics in the mushy region have high viscosity and at the same time set on the base of operations metal, with correspondingly lower spreading force. Fine grain size gives eutectics both increased strength and increased ductility. Highly accurate melting temperature lets joining process exist performed only slightly higher up the alloy's melting point. On solidifying, there is no mushy country where the alloy appears solid only is not yet; the chance of disturbing the joint by manipulation in such state is reduced (assuming the alloy did not significantly change its properties past dissolving the base metal). Eutectic behavior is especially benign for solders.[23]
Metals with fine grain structure before melting provide superior wetting to metals with large grains. Alloying additives (e.thousand. strontium to aluminium) tin be added to refine grain structure, and the preforms or foils can be prepared by rapid quenching. Very rapid quenching may provide amorphous metallic structure, which possess further advantages.[23]
Interaction with base metals [edit]

Brazing at the Gary Tubular Steel Institute, 1943
For successful wetting, the base metallic must be at to the lowest degree partially soluble in at least i component of the brazing alloy. The molten alloy therefore tends to attack the base metal and dissolve information technology, slightly irresolute its limerick in the process. The composition change is reflected in the modify of the alloy's melting point and the corresponding alter of fluidity. For example, some alloys dissolve both silvery and copper; dissolved silver lowers their melting indicate and increases fluidity, copper has the opposite effect.
The melting betoken change can be exploited. As the remelt temperature can be increased by enriching the alloy with dissolved base metal, pace brazing using the aforementioned braze tin can be possible.[27]
Alloys that do non significantly assault the base metals are more suitable for brazing thin sections.
Nonhomogenous microstructure of the braze may cause non-uniform melting and localized erosions of the base metal.[ citation needed ]
Wetting of base metals tin exist improved by calculation a suitable metal to the alloy. Tin facilitates wetting of iron, nickel, and many other alloys. Copper wets ferrous metals that silver does not attack, copper-silvery alloys can therefore braze steels silvery alone won't wet. Zinc improves wetting of ferrous metals, indium every bit well. Aluminium improves wetting of aluminium alloys. For wetting of ceramics, reactive metals capable of forming chemical compounds with the ceramic (east.g. titanium, vanadium, zirconium...) can exist added to the braze.
Dissolution of base metals can cause detrimental changes in the brazing blend. For example, aluminium dissolved from aluminium bronzes tin can embrittle the braze; improver of nickel to the braze can first this.[ commendation needed ]
The effect works both ways; there can be detrimental interactions between the braze alloy and the base metal. Presence of phosphorus in the braze alloy leads to formation of breakable phosphides of iron and nickel, phosphorus-containing alloys are therefore unsuitable for brazing nickel and ferrous alloys. Boron tends to diffuse into the base metals, especially forth the grain boundaries, and may form brittle borides. Carbon can negatively influence some steels.[ citation needed ]
Care must be taken to avert galvanic corrosion betwixt the affix and the base metal, and particularly between dissimilar base metals existence brazed together. Germination of brittle intermetallic compounds on the alloy interface tin cause joint failure. This is discussed more in-depth with solders.
The potentially detrimental phases may be distributed evenly through the book of the alloy, or be concentrated on the braze-base of operations interface. A thick layer of interfacial intermetallics is usually considered detrimental due to its commonly low fracture toughness and other sub-par mechanical properties. In some situations, e.g. die attaching, it however does not thing much every bit silicon chips are non typically subjected to mechanical abuse.[23]
On wetting, brazes may liberate elements from the base metallic. For case, aluminium-silicon braze wets silicon nitride, dissociates the surface so it tin can react with silicon, and liberates nitrogen, which may create voids forth the joint interface and lower its force. Titanium-containing nickel-gold braze wets silicon nitride and reacts with its surface, forming titanium nitride and liberating silicon; silicon then forms brittle nickel silicides and eutectic gilt-silicon stage; the resulting joint is weak and melts at much lower temperature than may be expected.[23]
Metals may lengthened from one base blend to the other ane, causing embrittlement or corrosion. An case is improvidence of aluminium from aluminium statuary to a ferrous alloy when joining these. A improvidence barrier, e.g. a copper layer (eastward.g. in a trimet strip), can be used.
A sacrificial layer of a noble metal can exist used on the base of operations metal every bit an oxygen barrier, preventing germination of oxides and facilitating fluxless brazing. During brazing, the noble metal layer dissolves in the filler metal. Copper or nickel plating of stainless steels performs the same function.[23]
In brazing copper, a reducing temper (or even a reducing flame) may react with the oxygen residues in the metal, which are present as cuprous oxide inclusions, and cause hydrogen embrittlement. The hydrogen nowadays in the flame or atmosphere at loftier temperature reacts with the oxide, yielding metallic copper and water vapour, steam. The steam bubbling exert high force per unit area in the metal structure, leading to cracks and joint porosity. Oxygen-free copper is not sensitive to this effect, even so the most readily bachelor grades, e.g. electrolytic copper or high-electrical conductivity copper, are. The embrittled joint may then fail catastrophically without any previous sign of deformation or deterioration.[28]
Preform [edit]
A brazing preform is a loftier quality, precision metal stamping used for a variety of joining applications in manufacturing electronic devices and systems. Typical brazing preform uses include attaching electronic circuitry, packaging electronic devices, providing good thermal and conductivity, and providing an interface for electronic connections. Square, rectangular and disc shaped brazing preforms are unremarkably used to attach electronic components containing silicon dies to a substrate such as a printed circuit lath.
Rectangular frame shaped preforms are often required for the structure of electronic packages while washer shaped brazing preforms are typically utilized to attach lead wires and hermetic feed-throughs to electronic circuits and packages. Some preforms are too used in diodes, rectifiers, optoelectronic devices and components packaging.[29]
•Deviation between soldering and brazing
Soldering involves joining of materials with a filler metal that melts below ~450 °C. Information technology generally requires a relatively fine and uniform surface finish between the faying surfaces. The solder joints tend to be weaker due to the lower forcefulness of the solder materials.
Brazing utilizes filler materials with a melting temperature to a higher place ~450 °C. Surface finish tends to be less critical and the affix joints tend to exist stronger.
See also [edit]
- Affix-on
- CuproBraze
- Petit chien à bélière
- Soldering
References [edit]
- ^ a b c d e Groover 2007, pp. 746–748
- ^ a b c Schwartz 1987, pp. 20–24
- ^ a b "Lucas-Milhaupt SIL-FOS 18 Copper/Silverish/Phosphorus Alloy". MatWeb – The Online Materials Information Resources.
- ^ Schwartz 1987, pp. 271–279
- ^ a b Schwartz 1987, pp. 131–160
- ^ Schwartz 1987, pp. 163–185
- ^ The Brazing Guide Archived April two, 2015, at the Wayback Machine. GH Consecration Atmospheres
- ^ Joseph R. Davis, ASM International. Handbook Commission (2001). Copper and copper alloys. ASM International. p. 311. ISBN0-87170-726-8. Archived from the original on 2017-02-27.
- ^ AWS A3.0:2001, Standard Welding Terms and Definitions Including Terms for Agglutinative Bonding, Brazing, Soldering, Thermal Cutting, and Thermal Spraying, American Welding Lodge (2001), p. 118. ISBN 0-87171-624-0
- ^ a b c d Schwartz 1987, pp. 189–198
- ^ a b c d due east f Schwartz 1987, pp. 199–222
- ^ "Vacuum Brazing of Aluminum Cold Plates and Estrus Exchangers – Lytron Inc". www.lytron.com . Retrieved 2017-12-27 .
- ^ "Flux Brazing Alloys | Lynch Metals, Inc". Lynch Metals, Inc . Retrieved 2017-12-27 .
- ^ Schwartz 1987, pp. 24–37
- ^ "FAQ: What are the different methods of brazing?". The Welding Institute . Retrieved 27 December 2017.
- ^ "CDC - NIOSH Publications and Products - Criteria for a Recommended Standard: Welding, Brazing, and Thermal Cutting (88-110)". www.cdc.gov. 1988. doi:10.26616/NIOSHPUB88110. Archived from the original on 2017-04-12. Retrieved 2017-04-11 .
- ^ "Joining Dissimilar Metals" Archived 2014-03-04 at the Wayback Machine. Deringer-Ney, Apr 29, 2014
- ^ Schwartz 1987, p. 3
- ^ Schwartz 1987, pp. 118–119
- ^ Alan Belohlav. "Understanding Brazing Fundamentals". American Welding Society. Archived from the original on 2014-02-27.
- ^ "Guidelines for Selecting the Right Brazing Blend". Silvaloy.com. Archived from the original on 2010-10-07. Retrieved 2010-07-26 .
- ^ a b c d e f g Christopher Corti; Richard Holliday (2009). Golden: Science and Applications. CRC Press. pp. 184–. ISBN978-1-4200-6526-8. Archived from the original on 2017-xi-01.
- ^ a b c d due east f g h i j one thousand l k north o p q r David 1000. Jacobson; Giles Humpston (2005). Principles of Brazing. ASM International. pp. 71–. ISBN978-1-61503-104-seven. Archived from the original on 2017-11-13.
- ^ a b Philip Roberts (2003). Industrial Brazing Practise. CRC Press. pp. 272–. ISBN978-0-203-48857-7. Archived from the original on 2017-11-13.
- ^ a b "Ag slitiny bez Cd – speciální aplikace". Archived from the original on 2016-04-20. Retrieved 2016-04-07 .
- ^ "Ceramic Brazing". Azom.com. 2001-xi-29. Archived from the original on 2008-08-21. Retrieved 2010-07-26 .
- ^ "Welding Vs Soldering Vs Brazing - Learn The Difference". Welder Selection. 2021-03-01. Retrieved 2021-03-03 .
- ^ Supplies of Cadmium Begetting Silver Solders Continue (2009-01-20). "Force of Silver Solder Joints". www.cupalloys.co.uk. Archived from the original on 2011-08-12. Retrieved 2010-07-26 .
- ^ Solder Preforms Archived July 8, 2011, at the Wayback Machine. AMETEK.Inc.
Bibliography [edit]
- Groover, Mikell P. (2007). Fundamentals Of Mod Manufacturing: Materials Processes, And Systems (2d ed.). John Wiley & Sons. ISBN978-81-265-1266-9.
- Schwartz, Mel M. (1987). Brazing. ASM International. ISBN978-0-87170-246-three.
Farther reading [edit]
- Fletcher, M.J. (1971). Vacuum Brazing. London: Mills and Boon Limited. ISBN0-263-51708-X.
- P.K. Roberts, "Industrial Brazing Practise", CRC Printing, Boca Raton, Florida, 2004.
- Kent White, "Accurate Aluminum Gas Welding: Plus Brazing & Soldering." Publisher: TM Technologies, 2008.
- Andrea Cagnetti (2009). "Experimental survey on fluid brazing in ancient goldsmith' fine art". International Journal of Materials Research. 100: 81–85. doi:10.3139/146.101783. S2CID 137786674.
External links [edit]
| | Wikimedia Commons has media related to Brazing. |
- European Association for Brazing and Soldering
Source: https://en.wikipedia.org/wiki/Brazing
Posted by: reeddrempan1965.blogspot.com


0 Response to "Which Copper-zinc Brazing Alloy Could Be Used To Join Or Repair Each Of The Following"
Post a Comment